The endoscope is by definition an optical, medical, or industrial instrument used for performing an endoscopy. Medical endoscopes allow doctors to view the inside of a patient’s body during intrusive surgical operations – even microsurgery. Specific to industry, endoscopes permit the observation of hard-to-access places.
There are different versions of endoscopes, ranging from rigid “boroscopes” to pliable “flexoscopes”, as well as “fiberscopes” which are comprised of a flexible bundle of optical fibers.
The emergence of miniaturized cameras has transformed the nature of endoscopes, which were once only optical but have now become videoscopes which also integrate a lighting solution.
PISÉO, an innovation platform in optics-photonics, provides characterization services for endoscopes that embed cameras – and more particularly, medical endoscopes. The tests carried out by PISÉO, which are done fully independently, are recognized and based on international standards. PISÉO’s tests make it possible to demonstrate regulatory compliance of tested devices, as well as to obtain certifications – for example, CE certification or FDA approval for the American market.

The measurements and tests carried out in the PISÉO imaging laboratory relate to the following characteristics, most of which are required for certification or approval:
- Measurement of the field of view (FoV) and the direction of the FoV relative to the distal glass of the endoscope: this measurement is carried out according to test method B of the ISO 8600-3 standard. The results are compared with the requirements of the ISO 8600-1 standard.
- Measurement of resolution and response of the endoscope to spatial frequencies: this measurement is carried out using either the internal lighting of the endoscope or an external light source (with the internal lighting of the endoscope being neutralized). The external light source meets the requirements of the ISO 12233 standard in terms of luminance uniformity and emission spectrum.
- Distortion measurement on a checkered pattern.
- Measurement of the signal-to-noise ratio (SNR): this measurement can be carried out at room temperature or in an oven at 37°C for medical endoscopes. In the latter case, the entire test device and the endoscope are placed inside a climatic chamber, without the need for opening it to carry out the measurement. Thermal balance is thus maintained throughout the duration of the test. The light source used to backlight the test pattern meets the ISO 12233 standard for the uniformity of its luminance and the ISO 7589 standard for its spectrum emission. The measurement results are presented according to the requirements of the ISO 15739 standard.
- Measurement of the uniformity of the gray levels of the image (“image intensity uniformity”): this measurement is done using a Lambertian diffusing screen, illuminated by the internal lighting of the endoscope and according to FDA recommendations.
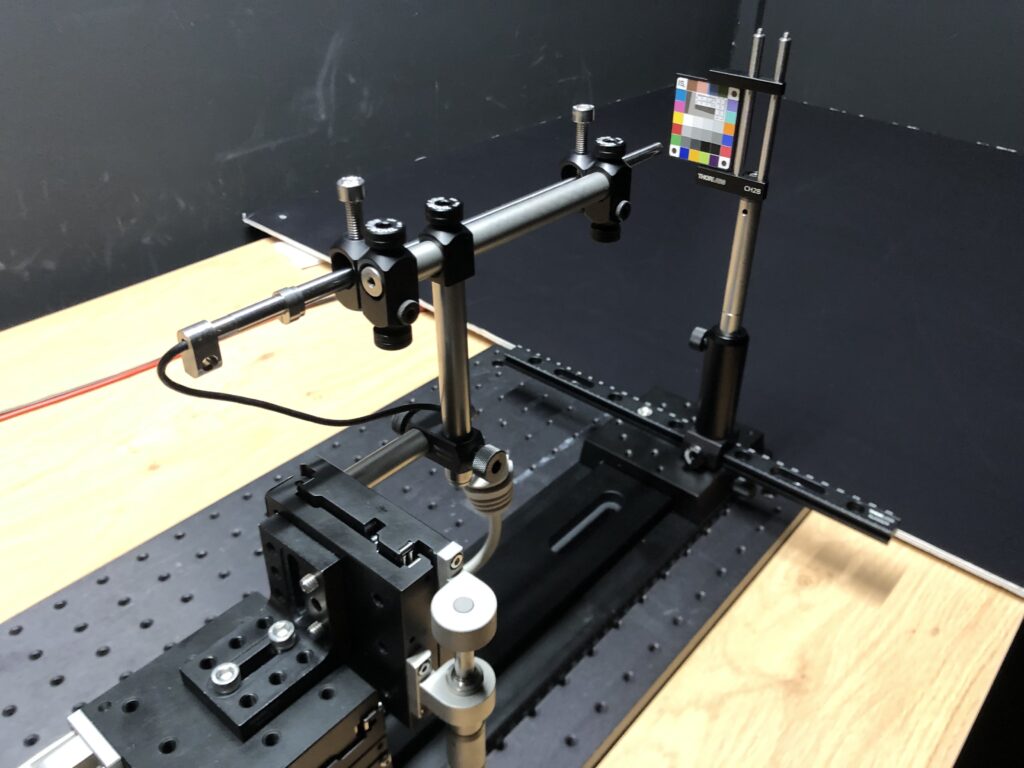
- Color rendering and colorimetry: colorimetry is measured on a Rezchcker target. The color differences on the colored areas of the test chart are measured and the DeltaE00 and DeltaC00 values are calculated.
To carry out our characterizations, PISÉO’s experts rely on recognized testing methods (international standards). Our team can also develop protocols and scripts in Python for carrying out specific tests. The list below presents a non-exhaustive list of standards mastered by our laboratory:
- ISO 12233: imaging of electronic images — resolution and spatial frequency responses
- ISO 15739: imaging of electronic images — noise measurements
- ISO 14524: electronic cameras — methods for measuring opto-electronic conversion functions
- ISO 7589: photography — sensitometric illuminants — specifications for daylight, artificial light, and printer
The PISÉO optical imaging laboratory is equipped with all of the equipment necessary to carry out the measurements mentioned above. The following list provides an overview:
- Image quality analysis software IMATEST v22.1
- Standard sights produced according to the ISO 12233 standard, or following a specific protocol defined with our client
- IMATEST light sources with luminance uniformity greater than 90%, in the visible range (color temperature 3100K)
- Binder climatic chamber, reference MK115