Many software applications dedicated to Augmented, Virtual and Mixed Reality (AR, VR and MR) systems have been developed to enable tools, particularly in maintenance and medical fields. These applications are optimized to exploit the functionalities available in AR, VR or MR systems, whether they are in the form of glasses or helmets, integrating a smartphone or not.
It is important to know the optical characteristics of an AR, VR or MR system to properly develop the software application that will be associated with it to obtain maximum performance.
For a customer in the medical field, PISÉO has developed a test protocol to know the actual optical performance of the AR system selected in connection with its software application, with a view to US Food and Drug Administration (FDA) certification for the American market. The measurements of the optical performance of these systems were carried out in the PISÉO test lab, in a controlled environment and with test equipment calibrated under ISO 17025 accreditation. They follow the recommendations given by the IEC 63145-20-20 standards and IDMS v1.1a.

AR for augmented reality makes it possible to superimpose digital content on the real world to introduce virtual objects and information. The user still sees the real environment, superimposed with this layer of digital content. The display takes place on a screen visible to the user, most often via augmented reality glasses or via a smartphone with a specific application offering AR capabilities.
Unlike AR, VR encompasses all the immersive experiences available via a VR headset or head-mounted display (HMD). The user is completely cut off from the real world, in a virtual environment visible through the VR headset.
Particularly used in the medical field to assist pre-operative teams, VR totally immerses the user into a 3D virtual environment via a VR headset. The user can thus turn their head 360° around themselves, move in space and interact with the virtual environment thanks to joysticks, for example.
VR is based on 3 pillars: interaction, immersion and the feeling of presence which work together to create a realistic virtual environment.
MR combines several technologies. An MR headset makes it possible to integrate virtual elements into the real world by allowing these elements to interact with the environment. The user is not cut off from the real world, they can see through the headset or mixed reality glasses.
This technology mixes AR and VR, which increases its potential compared to VR alone.
AR, VR or MR systems present themselves in different sectors. Some are connected to a PC and are controlled by a Windows MR type application, while others are autonomous. Still others use a smartphone with a dedicated application.
Various performance tests are performed on these systems, but many of them are performed with protocols that are poorly defined or not defined at all. These measurements are frequently carried out with poorly controlled and uncontrolled experimental conditions. The lack of rigor when carrying out these measurements or checks can also distort their results. Under these conditions, it is difficult to compare the systems marketed objectively. With its long experience in optical system performance measurement and its ISO 17025 accreditation, PISÉO can develop protocols to test AR and VR systems.
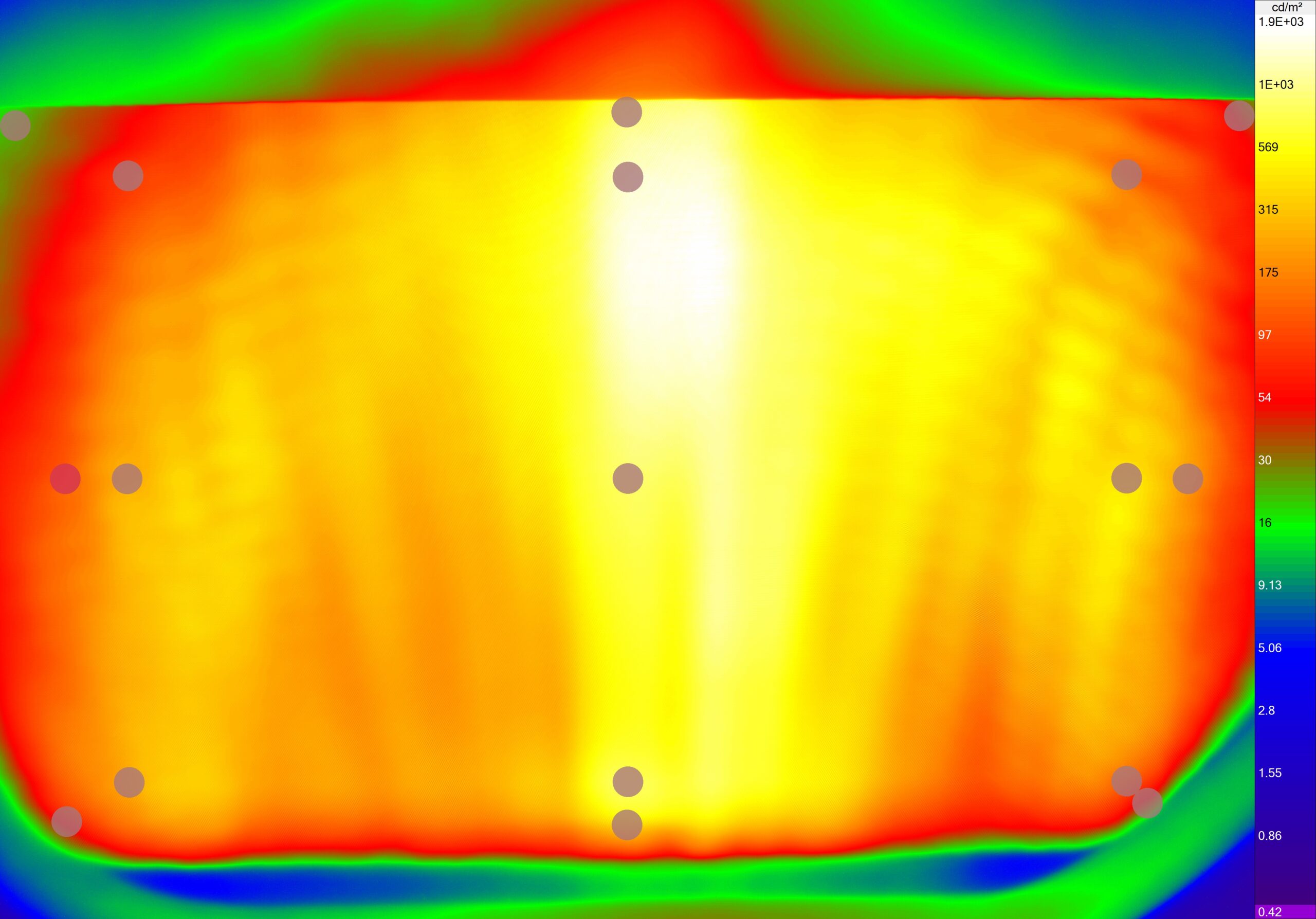
At the request of a customer in the medical field, performance tests were carried out on Microsoft Hololens2 mixed reality glasses in the PISÉO laboratory. These tests were the subject of a prior analysis of the FDA specifications and the definition of a rigorous repeatable and reproducible protocol. The latter is based on specific internationally recognized test methods and the practice of PISÉO image quality measurement. The tests carried out focused on the following aspects:
• The measurement of the spatial resolution of the glasses by measuring the Michelson contrast on vertical and horizontal grid patterns according to the recommendations of the IEC 63145-20-20:2019 standard. These measurements were carried out in the dark and for several levels of ambient light illumination. The light levels were generated by a PISÉO laboratory illuminator and measured by a PISÉO laboratory device calibrated under ISO 17025 accreditation.
• Contrast measurement on a checkerboard pattern according to IEC 63145-20-20:2019, for several levels of ambient light.
• Measurement of luminance and luminance uniformity according to standard IEC 63145-20-20:2019.
• Geometric distortion measurement according to IEC 63145-20-20:2019.
• Spatial persistence measurement for which the protocol was established by PISÉO. Spatial persistence is the difference in the speed of movement between a real object perceived through the Hololens2 glasses, and a virtual object projected on the screen of the glasses. If the spatial persistence is too much, it can make the user of Hololens2 uncomfortable.

Given the display technology of the Hololens2 screen using the scanning of a laser beam, it was necessary to adapt the acquisition frequency of the measuring camera or Light Measuring Device (LMD) to the scanning frequency of the laser beam.
Each of the measurements presented above was carried out on each of the eyepieces of the Hololens2 glasses.
The alignment of the LMD matrix camera was carried out according to the recommendations described in the chapter dedicated to the tests on the Near Eye Display (NED) of the IDMS v1 standard 1a.
The laboratory measurements allowed the customer to qualify different parameters of the Hololens and thus ensure the performance of their VR system. Ultimately, the tests carried out by PISÉO enabled the client to obtain FDA certification to sell its application on the American market.



